Atmospheric impacts of halogen monoxide reactions with organic peroxy radicals: Direct measurements of reaction kinetics and product yields
Halogen chemistry in the marine boundary layer (MBL) has significant impacts on climate through influences on budgets of ozone (O3), HOx (HOx = OH + HO2), and NOx (NOx = NO + NO2), as well as effects on aerosol formation. Understanding this chemistry is critical for the accuracy and reliability of atmospheric models used to assess and predict the impacts of our changing atmosphere on climate.
The production of bromine and iodine atoms in the MBL following emissions of organohalogen compounds and inorganic compounds such as Br2, I2, and HOI has been shown to result in considerable destruction of tropospheric ozone (Read et al., 2008), and leads to the production of the radicals bromine monoxide (BrO) and iodine monoxide (IO). In the MBL, halogen monoxides (XO, where X = halogen) react with hydroperoxy radicals (HO2) to produce hypohalaous acids (HOX) which photolyse during the day, releasing hydroxyl radicals (OH) and halogen atoms (X):
XO + HO2 → HOX + O2
HOX + hν → OH + X
The chemistry of halogen monoxides thus shifts the balance between OH and HO2 and impacts the lifetimes of climate gases such as methane (CH4), which are primarily removed from the atmosphere by reaction with OH. The impacts of halogen species on OH and HO2 have been demonstrated in a number of field and modelling studies (e.g. Whalley et al., 2010; Stone et al., 2018). However, recent measurements and modelling of radical species by the Leeds group in the MBL at the Cabo Verde Atmospheric Observatory (CVAO), which included the first simultaneous measurements of OH, HO2, organic peroxy radicals (RO2) and OH reactivity in the MBL, have indicated the potential importance of the chemistry between halogen monoxides and organic peroxy radicals (RO2). Despite the potential importance of XO + RO2 chemistry, our understanding and ability to assess the atmospheric impacts of this chemistry are hindered by uncertainties in reaction kinetics and product yields, particularly for IO + CH3O2:
IO + CH3O2 → products
Previous measurements of the kinetics of IO + CH3O2 vary by more than an order of magnitude at room temperature (Bale et al., 2005; Enami et al., 2006; Dillon et al., 2006; Dillon et al., 2010), and there has been only one study of the temperature dependence of the reaction kinetics (Enami et al., 2006). Expected impacts of the chemistry between IO and CH3O2 differ significantly between atmospheric models using rate coefficients at the upper and lower end of the range of measured values, and uncertainties are compounded by uncertainties in product yields, with possible product channels leading to production of methoxy radicals (CH3O) and OIO or IOO, or the Criegee intermediate formaldehyde oxide (CH2OO) and HOI.
In this work you will perform a detailed and comprehensive laboratory study of the kinetics and product yields of reactions between halogen monoxides and organic peroxy radicals, with a focus on IO + CH3O2.
You will use laser flash photolysis coupled with broadband time-resolved UV absorption spectroscopy (Figure 1, Lewis et al., 2018; Mir et al., 2020; Lade et al., 2024) to determine reaction kinetics over a range of atmospherically relevant conditions, including temperature, which will enable the simultaneous measurement of IO and CH3O2, as well as the potential products CH2OO and OIO.
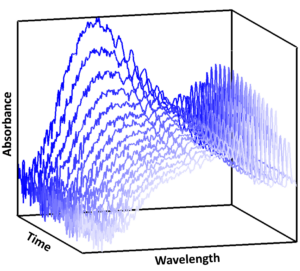
Figure 1: Time-resolved UV absorbance spectra used to monitor concentrations of reactants and products throughout the course of a reaction in real-time (Mir et al., 2020).
You will also have opportunities to investigate the production of CH3O radicals using laser-induced fluorescence (LIF) spectroscopy (Onel et al., 2017), and of other species using time-resolved mid-infrared quantum cascade laser absorption spectroscopy (Figure 2, Mir et al., 2022).
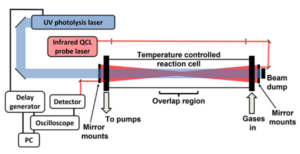
Figure 2: Laser flash photolysis coupled with time-resolved mid-infrared quantum cascade laser absorption spectroscopy (Mir et al., 2022).
You will assess the atmospheric impacts of your experimental results using atmospheric chemical models based on the Master Chemical Mechanism (MCM) (e.g. Figure 3, Whalley et al., 2010; Stone et al., 2018) and compared to field measurements of OH, HO2, and RO2 made in the MBL. There may also be opportunities for involvement in field measurements at the Cabo Verde Atmospheric Observatory.

Figure 3: Loss of hydroperoxy (HO2) radicals determined at the Cabo Verde Atmospheric Observatory using a numerical model based on the Master Chemical Mechanism (Stone et al., 2018).
Training
You will work under the supervision of Dr Daniel Stone, Dr Lisa Whalley, and Professor Paul Seakins from the School of Chemistry at Leeds, who are all members of the Atmospheric and Planetary Chemistry Group. The supervisors lead active and vibrant research groups exploring the role of gas-phase and aerosol chemical processes in the atmosphere, using experimental and modelling approaches.
You will work in well-equipped laboratories and be part of an active, thriving and well-funded atmospheric chemistry community. The Leeds group has an internationally leading reputation in atmospheric chemistry for field measurements of atmospheric composition, laboratory studies of chemical kinetics and photochemistry, and the development of numerical models and chemical mechanisms. Activities in these three areas are intimately linked and interdependent, providing significant advantages. You will be supported to attend both national and international conferences, and will receive a wide range of training, for example in communication skills, project management, and with other technical aspects. The PhD will provide a wide range of experience in the use of high power lasers, vacuum systems, optics, computer controlled data acquisition systems and methods in numerical modelling. You will also have access to training provided by the National Centre for Atmospheric Science. The successful PhD student will have access to a broad spectrum of training workshops that include managing your degree and preparing for your viva.

Your Profile
You should have an interest in atmospheric chemistry, air quality, climate and global environmental problems, with a strong background in chemistry or a similar discipline (e.g. natural sciences, environmental science, physics, engineering).
References
Bale et al., Physical Chemistry Chemical Physics, 7, 2164-2172, 2005
Dillon et al., Physical Chemistry Chemical Physics, 8, 5185-5198, 2006
Dillon et al., ChemPhysChem, 11, 4011-4018, 2010
Enami et al., Journal of Physical Chemistry A, 110, 9861-9866, 2006
Lade et al., Environmental Science: Atmospheres, 4, 1294-1308, 2024
Lewis et al., Review of Scientific Instruments, 89, 1-8, 2018
Mir et al., Physical Chemistry Chemical Physics, 22, 9448-9459, 2020
Mir et al., Atmospheric Measurement Techniques, 15, 2875-2887, 2022
Onel et al., Atmospheric Measurement Techniques, 10, 3985-4000, 2017
Read et al., Nature, 453, 1232-1235, 2008
Stone et al., 18, 5, 3541-3561, 2018
Whalley et al., Atmospheric Chemistry and Physics, 10, 4, 1555-1576, 2010
