Chlorine Atom Reactivity Measurements – CHARM
Chlorine Atom Reactivity Measurements – CHARM
Background and Context
The recent warning by the UN at COP30 that we are unlikely to meet the Paris Accord of limiting temperature rises to less than 1.5 oC by decreasing our emissions, increases the probability that we may need to consider geoengineering solutions. One of these is Enhanced Methane Removal (EMR). Methane, CH4, accounts for ~30% of anthropogenic radiation forcing and is removed relatively slowly by the main atmospheric oxidant, the hydroxyl, OH, radical. Atomic chlorine, Cl, reacts much faster and it has been suggested that promoting Cl production could significantly reduce atmospheric methane.1
One of the key issues around assessing the viability of EMR is our lack of understanding of the current contribution of Cl chemistry to tropospheric oxidation cycles. Specific questions include:
- What is the current contribution of Cl to methane removal?
- What fraction of enhanced Cl would react with methane?
- What fraction of Cl reacts with unsaturated hydrocarbons via addition and so introducing Cl atoms into the organic network?
Chlorine atom (Cl) chemistry has been known to be important in the marine boundary for some time, however, the observation of Cl precursors over continental landmasses mean that Cl chemistry may be more widespread.2 Although likely present in lower concentrations than other atmospheric oxidants, the much higher reactivity of Cl atoms means that Cl chemistry can be important. In addition, work from the University of York shows that Cl chemistry can be important in indoor air quality3, providing further impetus for study.
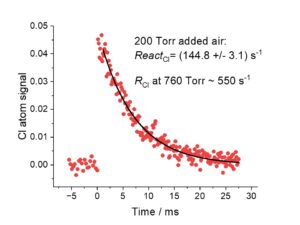
However, our understanding of the role of Cl is limited because there are no direct measurements of ambient Cl concentrations. As Cl atoms are so reactive they will be in a steady state where the rate of production (ROPCl) is equal to the rate of destruction (RODCl). At Leeds, we have recently developed a methodology for measuring the total rate of Cl removal in real air samples.4 Figure 1 shows an example of a Cl reactivity measurement (ReactCl) of air from outside the Chemistry Department. The RODCl = [Cl] x ReactCl. Therefore, in conjunction with measurements of ROPCl production from well-defined sources, we can now make accurate estimates of ambient Cl concentrations and hence start to assess the impact of Cl chemistry both indoors and out.
 |
 |
| Figure 1. Example of a typical reactivity measurement using air sampled from outside the Chemistry Department. | Figure 2. The HIRAC chamber. Provides an ideal test bed for reactivity testing. The University of York has a different chamber focusing on indoor air quality. |
Project Plan
The exact structure of the CHARM project depends on your particular interests. Further development of the current reactivity instrument and the construction of an alternative and complimentary method based on the comparative reactivity approach will be important components, but you will be able to shape the overall direction of the project. The project would involve:
1) Continued development and testing of Cl reactivity instrument. This would include tests using the HIRAC simulation chamber (Figure 2).
2) Indoor air quality measurements with the University of York (Dr Terry Dillon).
3) Development of alternative comparative reactivity measurements. These are cheaper and more readily deployable but need testing against absolute techniques.
4) Modelling work to assess the role of Cl chemistry in specific environments and applications, for example in exploring the validity of EMR.
The project will give you a wide range of practical and modelling skills to go along with the range of generic and employability skills that you will develop through the YES DTN.
Next Steps
If you are interested in this project, please do contact Prof Paul Seakins ([email protected]) for further information and to discuss your interests and how you would like to shape CHARM.
References
- Li, Q. Y.; Meidan, D.; Hess, P.; Añel, J. A.; Cuevas, C. A.; Doney, S.; Fernandez, R. P.; van Herpen, M.; Höglund-Isaksson, L.; Johnson, M. S., et al., Global Environmental Implications of Atmospheric Methane Removal through Chlorine-Mediated Chemistry-Climate Interactions. Nature Communications 2023, 14 (1).
- Thornton, J. A.; Kercher, J. P.; Riedel, T. P.; Wagner, N. L.; Cozic, J.; Holloway, J. S.; Dube, W. P.; Wolfe, G. M.; Quinn, P. K.; Middlebrook, A. M., et al., A Large Atomic Chlorine Source Inferred from Mid-Continental Reactive Nitrogen Chemistry. Nature 2010, 464 (7286), 271-274.
- Carslaw, N.; Aghaji, J.; Budisulistiorini, S. H.; Carslaw, D. C.; Chatzidiakou, L.; Cheung, R. W.; Dillon, T. J.; Edwards, P.; Genes, D.; Giorio, C., et al., The Ingenious Project: Towards Understanding Air Pollution in Homes. Environmental Science-Processes & Impacts 2025, 27 (2), 355-372.
- Blitz, M. A.; Speak, T. H.; Seakins, P. W., First Direct Observation of Equilibrium Involving Cl Atoms: Cl + C2H4 ↔ ClCH2CH2 by VUV Monitoring. J. Phys. Chem. A 2025, 129 (42), 9733-9744.
