Atmospheric Photolysis Rate Investigations in the Laboratory (APRIL)
Photochemistry controls a vast array of important processes, from astro- and atmospheric-chemistry to plasma technology and solar power. Despite the importance of the subject, technological limitations have inhibited studies of even simple gas-phase molecules. APRIL will exploit recent breakthroughs in technology, such as UV LEDs, VUV lasers and modern computational methods to study atmospheric “carbonyl” chemistry. Carbonyls (simple example, propanone) are used in a vast array of industrial applications, as solvents, pesticides, biofuels, and reagents for pharma, polymer and aroma chemicals. Many carbonyls are key to new “green” processes, increasing with importance under the net-zero agenda. Volatile carbonyls are ubiquitous in air, with direct emissions supplemented by in-situ production from oxidation of other trace gases. Atmospheric breakdown rates depend critically on radical availability. Carbonyl photolysis, at wavelengths (290 – 340 nm) where UV light is abundant, leads to a host of products, including free-radicals. A detailed understanding of carbonyl chemistry is therefore critical to quantifying radical production in air. These radicals are responsible for generation of harmful secondary pollutants such as ozone and particulate matter, with impacts on chemistry, climate and health.
A critical lack of photochemical data leads to large uncertainties in our understanding of atmospheric chemistry. Current state-of-science models use data from a model compound (butanone) to calculate photolysis rates for no less than eight further important carbonyls 1. In recent work (Figure 1) we demonstrated the precarious nature of these approximations.
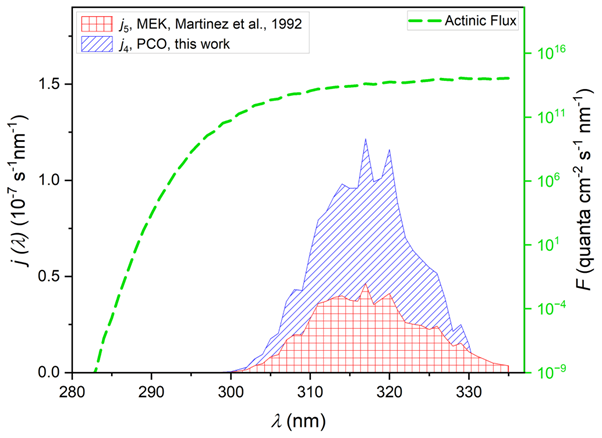
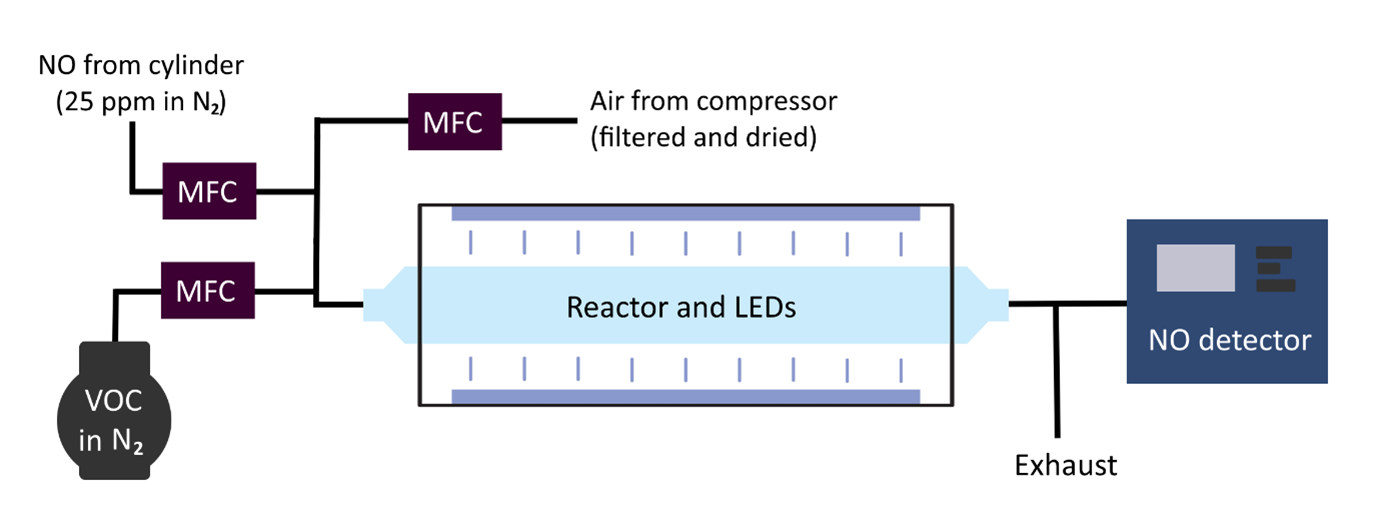
Accordingly, the objectives of this PhD are to: 1) determine reliable photolysis rates and products for simple carbonyls; 2) identify structural features controlling photochemical activity; 3) update models and assess carbonyl photolysis impacts on air quality. Key experiments: fast flow reactors equipped with LED photolysis and chemiluminescence detection (Figure 2); pulsed laser photolysis with laser induced fluorescence radical detection; quasi gas-phase UV-vis. spectroscopy 3. Computational: TD-DFT; TUV models; AtChem online 1.
You will be based in the Wolfson Atmospheric Chemistry Laboratories 4, at University of York and will benefit from the experience and expertise of the combined York and Leeds laboratory teams.
References:
- The master chemical mechanism mcm.york.ac.uk
- Mapelli et al. Atmos. Chem. Phys. (2023). doi.org/10.5194/acp-23-7767-2023
- Metcalf et al. RSC Sustainability (2025). In press.
- The Wolfson Atmospheric Chemistry Laboratories, WACL
