Real-time direct detection of Criegee intermediates formed by ozonolysis of alkenes in an atmospheric simulation chamber
Atmospheric oxidation chemistry is central to understanding air quality and climate. Ozonolysis reactions, in which ozone initiates the oxidation of unsaturated volatile organic compounds (VOCs) through addition to C=C double bonds, play a significant role in the atmosphere as a means of VOC removal and as a source of other reactive species and oxidants including Criegee intermediates (R2COO), hydroxyl radicals (OH), and peroxy radicals (HO2 and RO2) (Johnson & Marston, 2008; Donahue et al., 2011). The ozonolysis mechanism, and the subsequent chemistry initiated by the species it produces, leads to complex cascades of reactions which impact air quality and climate through the removal of primary pollutants and the production of secondary pollutants, and are important in regions impacted by both biogenic and anthropogenic emissions (Percival et al., 2013; Taatjes et al., 2014; Khan et al., 2018).
The Criegee intermediates produced in ozonolysis reactions have high internal energy and undergo either rapid unimolecular decomposition or collisional stabilisation with surrounding molecules to produce stabilised Criegee intermediates (SCIs) which may also undergo unimolecular decomposition or take part in bimolecular reactions with species such as water vapour, water vapour dimers, organic acids, and SO2. Although the role of Criegee intermediates in ozonolysis reactions was first suggested in 1949 (Criegee & Wenner, 1949) and the potential involvement of SCIs in the atmospheric oxidation of SO2 was highlighted in 1971 (Cox & Penkett, 1971a; Cox & Penkett, 1971b), challenges associated with direct studies on Criegee intermediate chemistry have hindered full understanding of their atmospheric impacts.
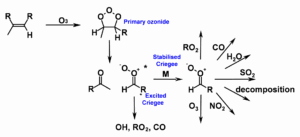
Figure 1: Production of Criegee intermediates in the atmosphere and their potential fates. Uncertainties in the yields, reaction kinetics, and products of stabilised Criegee intermediate reactions hinder our understanding of their impacts on atmospheric composition, air quality and climate.
The development of photolytic laboratory sources of certain SCIs in 2012 demonstrated unexpectedly high SCI reactivity (Welz et al., 2012), and has sparked a multitude of laboratory, theoretical, and modelling investigations, including work in this laboratory (eg. Mir et al., 2020; Robinson et al., 2022; Lade et al., 2024; Lade et al., 2025), which have led to significant improvements in our understanding of the SCI atmospheric chemistry and impacts (Cox et al., 2020). However, there is a limit to the SCIs that can be produced photolytically, with photolytic sources for many atmospherically relevant SCIs yet to be reported and likely to remain a challenge. Moreover, photolytic sources of SCIs bypass the complex dynamics and collisional stabilisation that occur during atmospheric ozonolysis, the natural formation route for SCIs, and provide no details regarding yields of SCIs from ozonolysis reactions. To address this problem, we have recently developed the use of UV cavity enhanced absorption spectroscopy (CEAS) to make real-time direct measurements of absolute concentrations of the CIs during ozonolysis reactions in an atmospheric simulation chamber. In this project you will use the CEAS technique to make direct measurements of SCI yields and reaction kinetics produced from a range of unsaturated VOCs relevant to our understanding of air quality and climate. You will also make use of the wide range of instruments coupled to the simulation chamber to support the CEAS measurements, with available techniques including Fourier transform infrared (FT-IR) spectroscopy, laser-induced fluorescence (LIF) spectroscopy, and mass spectrometry (Glowacki et al., 2007).
Your work will overcome a critical limitation – the absence of direct in situ measurements of SCIs formed by ozonolysis, the dominant atmospheric formation mechanism, under atmospheric conditions. Your work will provide a crucial link between photolytic studies of SCI chemistry and previous indirect measurements made via observations of stable products in ozonolysis-based approaches. Furthermore, your work will lay the foundation for conformer-resolved studies of SCIs in ozonolysis systems, the significance of which was first highlighted in 2013 (Taatjes et al., 2013) but has thus far only been explored in photolytic systems.
There is broad potential for application of CEAS to studies of SCI yields in ozonolysis reactions, which to date have only been possible via indirect methods, and of Criegee intermediate chemistry, particularly where photolytic sources are not available. CEAS offers both specificity and sensitivity, and the potential for determination of conformer-dependent SCI yields which are currently lacking in atmospheric models but can have potentially significant impacts where SCI conformers display differences in reactivity and product yields. Direct measurements of SCI yields and kinetics will provide further constraints to the development of the complex reaction mechanisms used to model atmospheric chemistry and composition, and thus to understand and predict air quality and climate.
Objectives
You will develop capabilities for real-time direct measurements of reactive species in laboratory experiments and atmospheric simulation chamber studies using time-resolved broadband absorption spectroscopy, providing valuable information on the yields, kinetics, and mechanisms of Criegee intermediates for assessments of atmospheric composition.
Training
The student will work under the supervision of Professor Daniel Stone and Professor Paul Seakins within the Atmospheric, Planetary, and Theoretical Chemistry group in the School of Chemistry at the University of Leeds. You will work in well-equipped laboratories and be part of an active, thriving and well-funded atmospheric chemistry community. The Leeds group has an internationally leading reputation in atmospheric chemistry for field measurements of atmospheric composition, laboratory studies of chemical kinetics and photochemistry, and the development of numerical models and chemical mechanisms. Activities in these three areas are intimately linked and interdependent, providing significant advantages. You will be supported to attend both national and international conferences, and will receive a training that includes areas such as communication and presentation skills, project management, and data science, in addition to the technical aspects of the project. The PhD will provide a wide range of experience in optical spectroscopy, the use of lasers, vacuum systems, computer controlled data acquisition systems, and methods in numerical modelling. You will also have access to training provided by the National Centre for Atmospheric Science.
References
